Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study
Notes by David Straton
The STAR*D project is a 6-year, $35 million study examining "next best" steps for patients with major depressive disorder who do not benefit from initial and subsequent treatments.
Diagramatic Summary

Flow diagram of research protocol
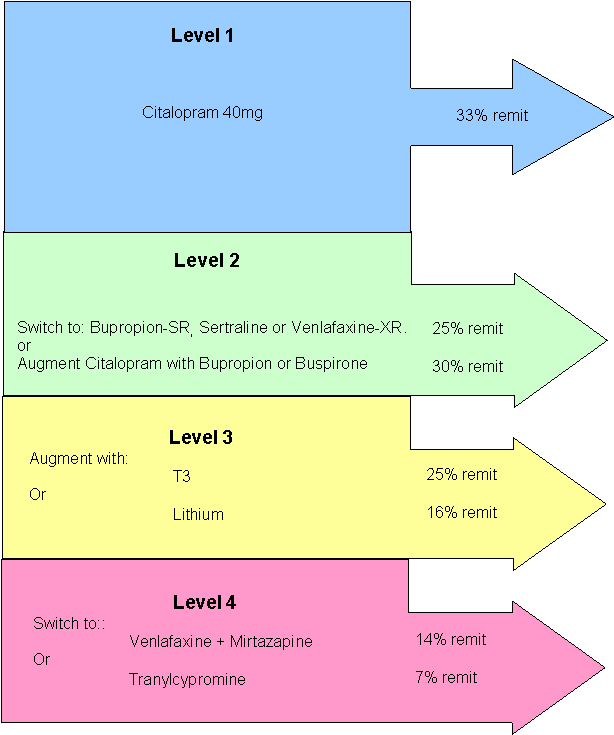
Level 1
Evaluation of Outcomes With Citalopram for Depression Using Measurement-Based
Care in STAR*D: Implications for Clinical Practice.
American Journal of Psychiatry 163:28-40, January 2006. Full
text. Pdf.
2,876 patients with Major Depression were treated with Citalopram for 12-14 weeks. Mean dose 41.8 mg/day.
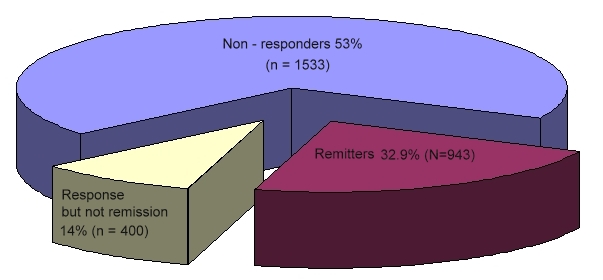
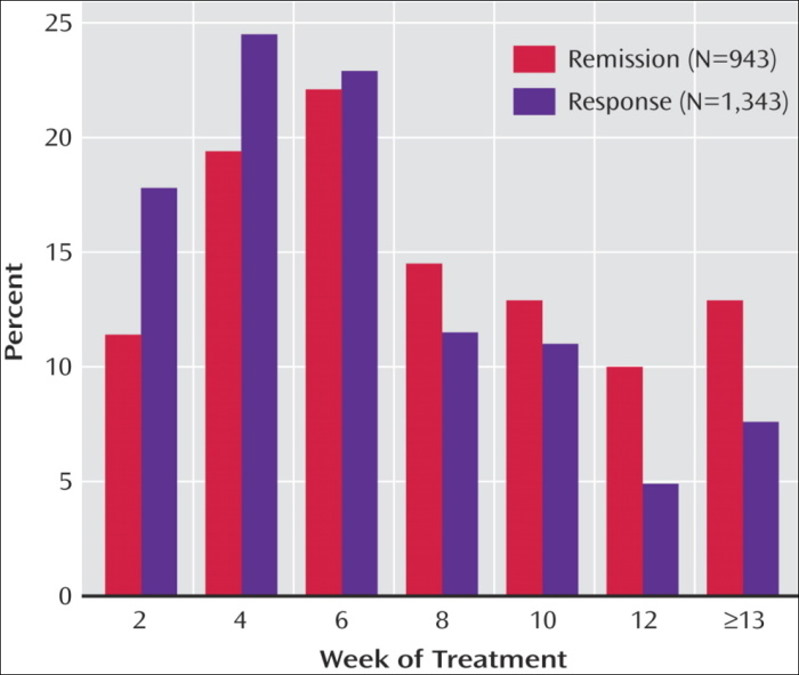
The overall remission rate was 27.5% (N=790) with the HAM-D definition (primary outcome) and 32.9% (N=943) with the QIDS-SR definition. The overall QIDS-SR response rate was 47% (N=1,343)
Surprises:
Level 2
In the Level 1 study, participants were evaluated for their depression outcomes from treatment with citalopram. Approximately one-third of those participants reached remission and if they chose to stay in the study, they continued on citalopram and entered a citalopram follow-up phase. Approximately two-thirds did not achieve a remission, and were eligible to enter Level 2. A total of 1,439 people chose to participate in Level 2.
Level 2 of the study offered seven different treatments; four options "switched" the study participants from citalopram to a new medication or talk therapy, and three options "augmented" citalopram treatment by adding a new medication or talk therapy to the citalopram they were already receiving.
Switch Option.
Bupropion-SR, Sertraline, or Venlafaxine-XR after Failure of SSRIs for Depression
New England Journal of Medicine. Volume 354:1231-1242 March 23, 2006 Number
12. Full-text.
727 adult outpatients with a nonpsychotic major depressive disorder who had no remission of symptoms or could not tolerate the SSRI citalopram were treated with one of the following drugs for up to 14 weeks: Bupropion-SR (239 patients) at a maximal daily dose of 400 mg, Sertraline (238 patients) at a maximal daily dose of 200 mg, or Venlafaxine-XR (250 patients) at a maximal daily dose of 375 mg. The primary outcome was symptom remission, defined by a total score of 7 or less on the 17-item Hamilton Rating Scale for Depression (HRSD-17) at the end of the study. Scores on the Quick Inventory of Depressive Symptomatology — Self Report (QIDS-SR-16), obtained at treatment visits, determined secondary outcomes, including remission (a score of 5 or less at exit) and response (a reduction of 50 percent or more on baseline scores).
Remission rates as assessed by the HRSD-17 and the QIDS-SR-16, respectively, were 21.3 percent and 25.5 percent for sustained-release bupropion, 17.6 percent and 26.6 percent for sertraline, and 24.8 percent and 25.0 percent for extended-release venlafaxine. QIDS-SR-16 response rates were 26.1 percent for sustained-release bupropion, 26.7 percent for sertraline, and 28.2 percent for extended-release venlafaxine. These treatments did not differ significantly with respect to outcomes, tolerability, or adverse events.
Note that there was a free choice for patients whether to enter the Switch arm or the Augmentation arm. The choice was thus not random or controlled. One cannot therefore conclude that augmenting is better than switching.
Switch: Citalopram stopped. New drug started instead.

Surprises:
Augmentation Option
Medication Augmentation after the Failure of SSRIs for Depression
New England Journal of Medicine. Volume 354:1243-1252 March 23, 2006 Number
12. Full-text
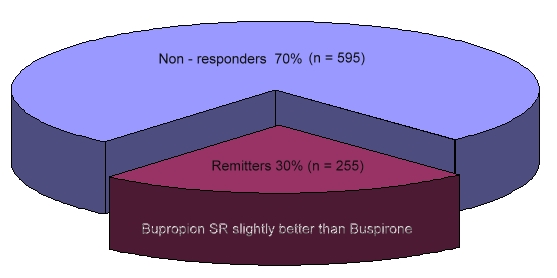
565 adult outpatients who had nonpsychotic major depressive disorder without remission despite a mean of 11.9 weeks of citalopram therapy (mean final dose, 55 mg per day) received Bupropion-SR (at a dose of up to 400 mg per day) as augmentation, and 286 received Buspirone (at a dose of up to 60 mg per day) as augmentation (as well as the Citalopram). The primary outcome of remission of symptoms was defined as a score of 7 or less on the 17-item Hamilton Rating Scale for Depression (HRSD-17). The 16-item Quick Inventory of Depressive Symptomatology — Self-Report (QIDS-SR-16) was used to determine the secondary outcomes of remission (defined as a score of less than 6 at the end of this study) and response (a reduction in baseline scores of 50 percent or more).
The Bupropion group and the Buspirone group had similar rates of HRSD-17 remission (29.7 percent and 30.1 percent, respectively), QIDS-SR-16 remission (39.0 percent and 32.9 percent), and QIDS-SR-16 response (31.8 percent and 26.9 percent). Bupropion, however, was associated with a greater reduction (from baseline to the end of this study) in QIDS-SR-16 scores than was Buspirone (25.3 percent vs. 17.1 percent, P<0.04), a lower QIDS-SR-16 score at the end of this study (8.0 vs. 9.1, P<0.02), and a lower dropout rate due to intolerance (12.5 percent vs. 20.6 percent, P<0.009).
Augmentation with sustained-release bupropion does have certain advantages, including a greater reduction in the number and severity of symptoms and fewer side effects and adverse events.
Augmentation: Citalopram continued. New drug added.
Level 3
A Comparison of Lithium and T3 Augmentation Following Two Failed Medication
Treatments for Depression: A STAR*D Report
American Journal of Psychiatry 163:1519-1530, September 2006. Full
text. Pdf.
142 adult outpatients with nonpsychotic major depressive disorder who had not achieved remission or who were intolerant to an initial prospective treatment with citalopram and a second switch or augmentation trial were randomly assigned to augmentation with Lithium (up to 900 mg/day; N=69) or with Triiodothyronine (T3) (up to 50 µg/day; N=73) for up to 14 weeks. The primary outcome measure was whether participants achieved remission, which was defined as a score =7 on the 17-item Hamilton Depression Rating Scale.
After a mean of 9.6 weeks of treatment, remission rates were 15.9% with lithium augmentation and 24.7% with T3 augmentation, although the difference between treatments was not statistically significant. Lithium was more frequently associated with side effects (p=0.045), and more participants in the lithium group left treatment because of side effects (23.2% versus 9.6%; p=0.027).
Surprise: The benefits were pretty modest, but T3 augmentation seems preferable to Lithium augmentation.
Level 4
Tranylcypromine Versus Venlafaxine Plus Mirtazapine Following Three Failed
Antidepressant Medication Trials for Depression: A STAR*D Report
American Journal of Psychiatry 163:1531-1541, September 2006. Full
text Pdf.
109 adult outpatients with nonpsychotic major depressive disorder who had not achieved remission or had withdrawn from treatment because of intolerance in three previous prospective medication trials were randomly assigned to receive open-label treatment with either Tranylcypromine (N=58) or Venlafaxine-XR plus Mirtazapine (N=51). The primary outcome measure was whether patients achieved remission, which was defined as a score =7 at exit on the 17-item Hamilton Depression Rating Scale (HAM-D).
Remission rates were not significantly different between the two treatment groups (6.9% for the tranylcypromine group and 13.7% for the venlafaxine plus mirtazapine group). The mean daily dose at exit for tranylcypromine was 36.9 mg (SD=18.5); for venlafaxine, 210.3 mg (SD=95.2); and for mirtazapine, 35.7 mg (SD=17.6). Tranylcypromine was associated with significantly less symptom reduction and greater attrition due to intolerance.
Surprises:
Discussion
Keeping Our Eyes on STAR*D
American Journal of Psychiatry 163:1484-1486, September 2006. Full
text. Pdf.
The STAR*D study: Treating depression in the real world
Gaynes BN, Wisniewski SR, Rush AJ, Spencer D, Trivedi MH, and Fava M.
Cleveland Clinic Journal of Medicine. Vol. 75; 1: 57-66 2008. Full
text pdf.
Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One
or Several Treatment Steps: A STAR*D Report
John Rush, et al. Am J Psychiatry 163:1905-1917, November 2006. Full
text pdf
STAR*D: What have we learned?
A. John Rush, M.D.
Am J Psychiatry 164:201-204, February 2007. Full
text pdf.