Thinking about Antidepressants
David Straton, MB ChB, DPM, FRANZCP
Between 2008 and 2018, there have been several publications which increase our understanding of antidepressants, their risks and benefits. This site brings together some of those papers in the hope that psychiatrists and their patients will find it helpful when deciding whether to use an antidepressant, and if so, which one.
The major studies were:
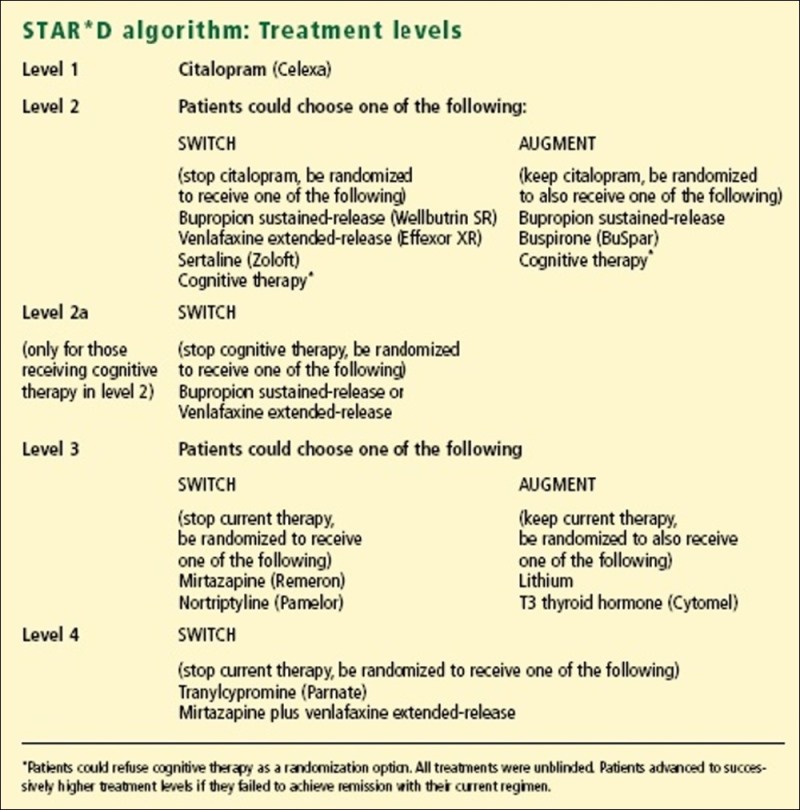
STAR*D (Sequenced Treatment Alternatives
to Relieve Depression Study)
See here for more detail
Very often, studies on anti-depressants are carried out on carefully selected research patients, chosen for not having any complications. The studies are commonly sponsored by the drug company producing the drug, and there is some concern that negative findings do not get published as often as positive ones, thus distorting the impression of a drugs value.
The STAR*D study used 'real-world' patients from across the USA, in both GP and specialist psychiatry settings. The numbers were large, although as the number of choices was also large, the numbers of cases at the later stages of the study were too small to be statistically significant.

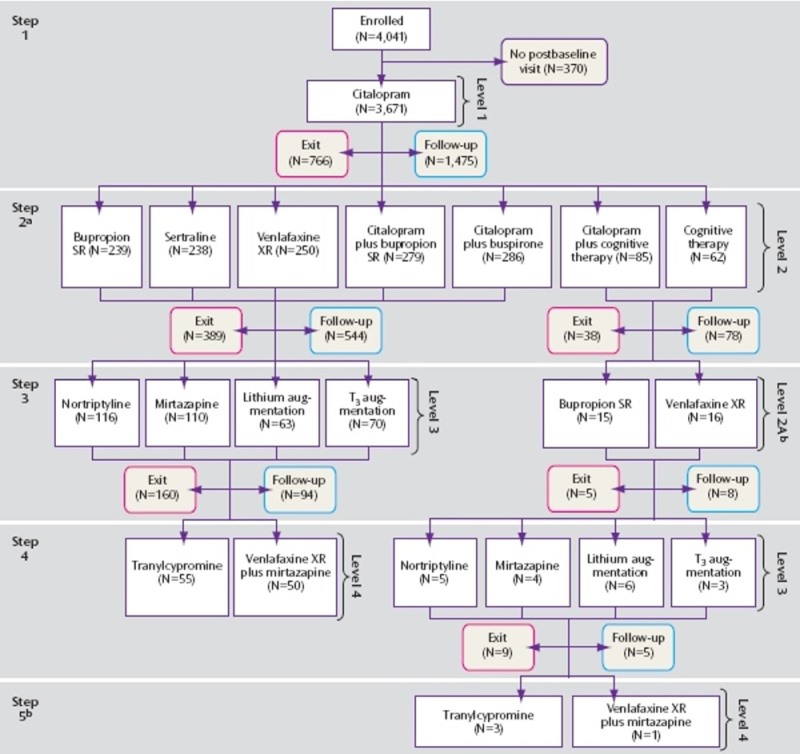
As can been seen, the numbers at the lower levels were too small for confident conclusions to be drawn.
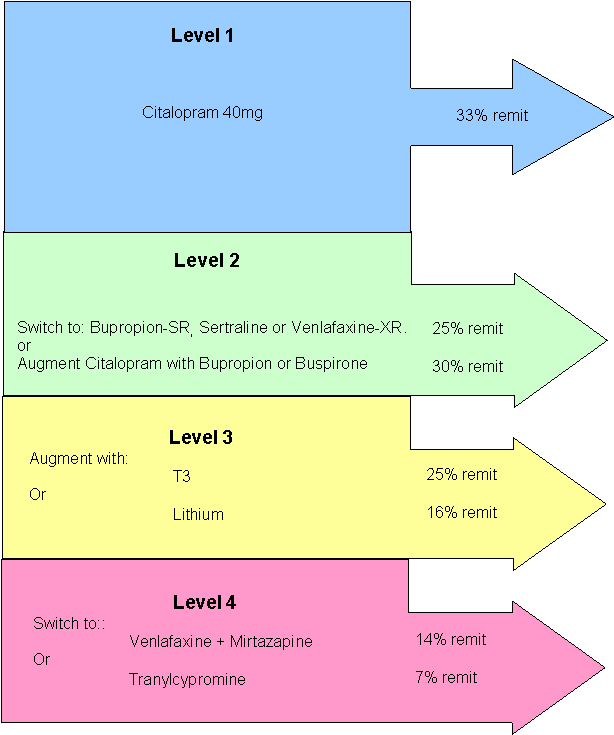
Summary of Results
Comment
Level 1
The mean dose of citalopram 40mg was higher than many clinicians use.
Participants who were Caucasian, female, employed, or had higher levels of education
or income had better remission rates.
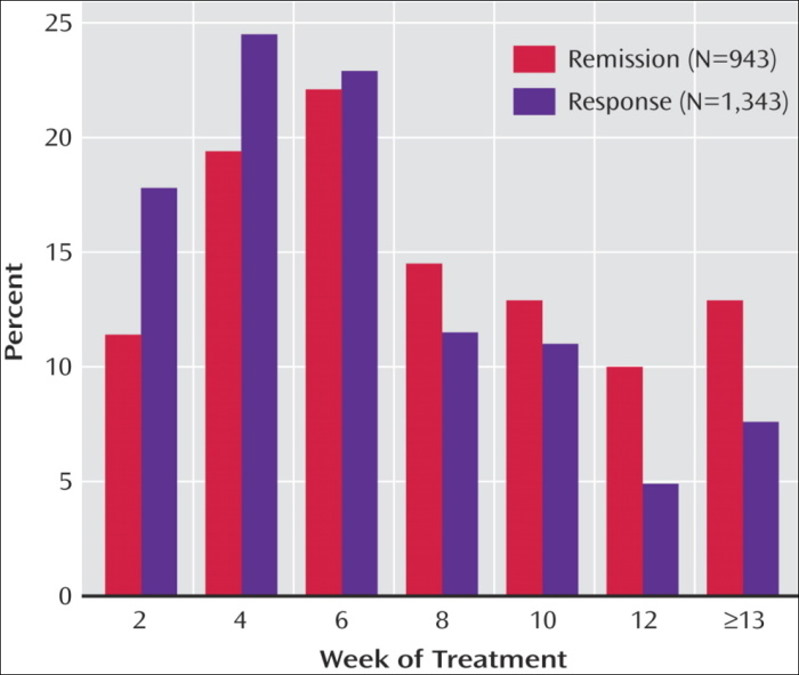
Of participants who responded, 56.0% did so only at or after 8 weeks of treatment.
The strong message was to persevere.
Level 2
Sertraline is an SSRI, which inhibits serotonin reuptake. This is the same class of medication as the SSRI citalopram used in the first treatment step and is therefore a "same class switch." Bupropion represents a medication from a different class, one that does not appear to have any direct effect on serotonin, and is referred to as an "out of class switch." Venlafaxine is a medication that acts on two neurotransmitters, inhibiting both serotonin and noradrenaline reuptake, and is referred to as a "dual action" agent. Although it has been a common assumption that a within-class switch after one antidepressant medication does not work well or might not be as effective as another approach, these findings suggest that any of these three approaches are reasonable choices. Finally, the dual action medication, venlafaxine, was not any more effective as a second step treatment than the other medications.
Level 3
The benefits were pretty modest, but T3 augmentation seems preferable to Lithium
augmentation 4.
Since T3 is converted into T4 (thyroxine), and is cheaper, it is a viable alternative.
Interestingly, the researchers did not report measurements of thyroid function
before or after augmenting with T3. One might imagine that the benefits of thyroid
augmentation might be greater for those patients whose T3 and T4 levels were
relatively low, and that the adverse effects might be worse for those whose
levels were near the top of the range.

Among all those who eventually remitted, 22% in the lithium group and 28% in the T3 group did not reach remission until week 14.
Level 4
This was the first formal evaluation of the mix of Mirtazapine and Venlafaxine, sometimes known as 'California rocket-fuel'. It beat Tranylcypromine, although the latter had a disadvantage. Patients on this arm of the trial had to have a two week 'washout', as a result of which 30% of the patients had less than two weeks of treatment. Not a fair go.
The cumulative remission rate for the first four steps were:
Level 1: 33%
Level 2: 57%
Level 3: 63%
Level 4: 67%
Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data
Submitted to the Food and Drug Administration
Kirsch I, et al. PLoS Med February 26, 2008, 5(2): e45 Full
text pdf
There has been serious concern about publication bias. This is where studies with positive results are more likely to be published than those with negative results, especially if the study is funded by a body with a financial interest in the result, such as the company making the drug. The Hull meta-analysis attempted to avoid this error by obtaining data on all clinical trials submitted to the FDA, both published and unpublished, on six modern anti-depressants, fluoxetine, venlafaxine, nefazodone, paroxetine, sertraline, and citalopram. Most of the 47 trials were of 6 weeks duration only. (Note this when reading the STAR*D results).
Five trials failed to show a statistically significant drug effect, and also failed to report mean HDRS scores. If these had been omitted, it might have led to a reporting bias, so the drugs (sertraline and citalopram) for which these studies showed no benefit, but reported no data, were left out of the meta-analysis. The omissions represented 38% of patients in sertraline trials, and 23% of patients in citalopram trials. (Note this when reading the Lancet meta-analysis, in which sertraline and citalopram score in the top five for both efficacy and acceptability. Might that be partly because the companies manged to keep the negative data out of the meta-analysis?).
The authors of the Hull meta-analysis conclude that the difference between drug and placebo is insignificant, citing NICE as specifying a three point change in the HRSD between drug and placebo as being the criterion of clinical significance. Reworking Table 1 data, shows that by pooling the numbers across all trials for a particular drug, compared to their placebo groups, produces these results.

It can be seen that venlafaxine and paroxetine pass the 3 point threshold, whereas fluoxetine and nefazadone do not. (Note this when reading the Lancet efficacy figures below).
The Hull authors conclude:
'Drug–placebo differences in antidepressant efficacy increase as a function of baseline severity, but are relatively small even for severely depressed patients. The relationship between initial severity and antidepressant efficacy is attributable to decreased responsiveness to placebo among very severely depressed patients, rather than to increased responsiveness to medication.'
I find this idea a bit confusing. If Dave is taller than Steve, is that due to Dave's height, or Steve's shortness?
There is another curious statement in the Editor's Summary.
'SSRIs are the newest antidepressants and include fluoxetine, venlafaxine, nefazodone, and paroxetine.'
Of course venlafaxine and nefazadone are not SSRIs. It makes one wonder about the attention to detail in the paper generally.
This paper is discussed here. Publication Bias and the Efficacy of Antidepressants
A further response in Dec 2011. Assessing the ‘true’ effect of active antidepressant therapy v. placebo in major depressive disorder: use of a mixture model
The American College of Physicians Reviews
See here for more detail
There are two papers, available here.
The second is more interesting for information about the relative merits of different antidepressants.
Comparative Benefits and Harms of Second-Generation Antidepressants:
Background Paper for the American College of Physicians.
Gerald Gartlehner, et al. Ann Intern Med. 2008 Nov 18;149(10):734-750. Full
text pdf.
This paper hedged it's bets on efficacy, 'Overall, we found no substantial differences in comparative efficacy and effectiveness of second-generation antidepressants for treatment of MDD'. On the other hand it did differentiate between modern antidepressants as regards to acceptability. Looking through these data, I think there is good reason to abandon fluvoxamine.
Other findings, amongst many inconclusive ones:
Although the authors bend over backwards to say that there are no clear differences between the different drugs they review, and their results generally support that, fluvoxamine appears to be the worst among the modern antidepressants.
See the evidence here. The case against fluvoxamine.
Lancet review of antidepressants
Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Andrea Cipriani, et al. Published online 29 January 2009. Full-text here.
This utilized a multiple-treatments meta-analysis, which accounts for both direct and indirect comparisons, to assess the effects of 12 new-generation antidepressants on major depression.

It expressed judgments about the relative efficacy and acceptability of different drugs, more clearly than the ACP papers.
Efficacy The cumulative probabilities of being among the four most effective treatments were:
|
Acceptability The cumulative probabilities of being among the four most acceptable treatments were:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Efficacy |
Acceptibility Higher is better |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ranking for efficacy (solid line) and acceptability
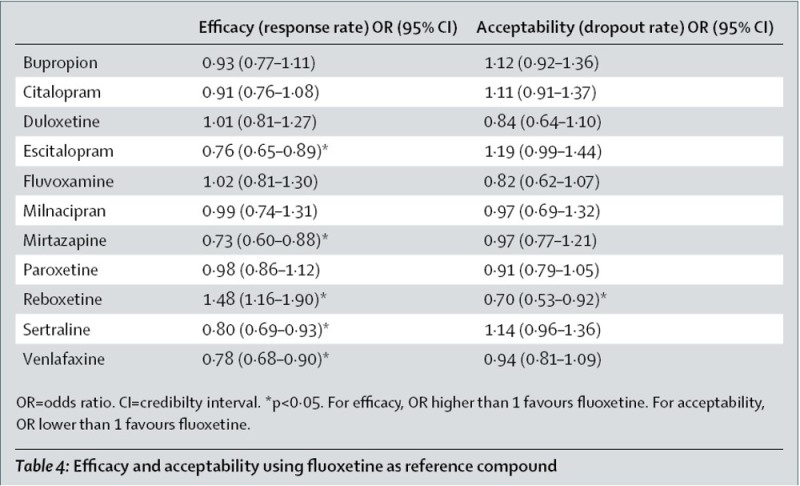
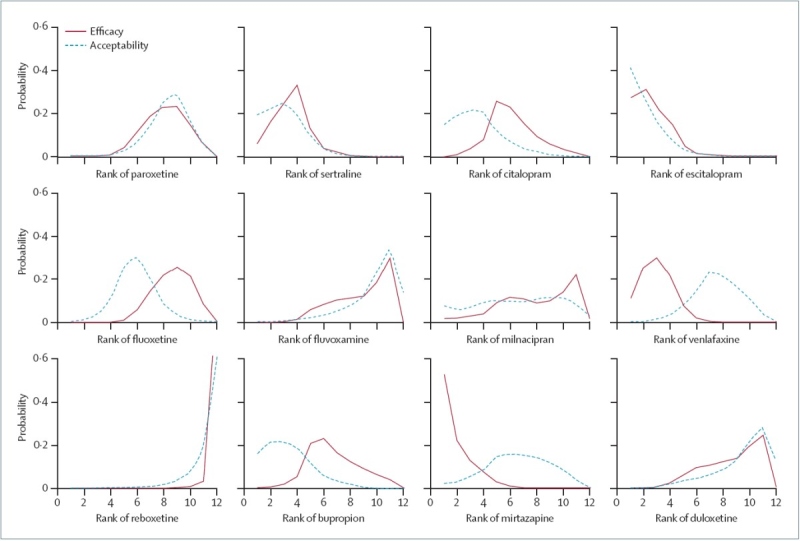
(dotted line). Lines over on the left are better. This makes the winners (escitalopram, sertraline, mirtazapine, and venlafaxine) obvious, but also illustrates how badly reboxetine and fluvoxamine do. I was surprised at the apparent difference between venlafaxine and duloxetine, which have seemed very similar to me, with, if anything, duloxetine having the advantage. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Suicide Risk Selective serotonin reuptake inhibitors and
risk of suicide: a systematic review of observational studies 'We found that the relation between exposure to SSRIs and the risk of suicide is influenced by age. Exposure to SSRIs decreased the risk of suicide by over 40% among adults, and decreased the risk by over 50% among elderly people. However, among adolescents, exposure to SSRIs almost doubled the risk of suicide.'
The authors attempted to see if some antidepressants were more risky of suicide than others.
Figure 4: Random-effect meta-analysis of the risk of suicide attempt and completion associated with the use of individual antidepressants compared with no exposure to any antidepressants. They comment: 'Paroxetine and venlafaxine may be better avoided based on the increasing evidence from randomized and observational studies that the risks might outweigh the benefits for most adolescents.' There is some literature suggesting that the self-harm impulses associated with SSRI use may be related to 5-HT2 receptors. 10 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sexual Side-effects Treatment-Emergent Sexual Dysfunction Related to Antidepressants:
A Meta-Analysis This table shows the relative size of sexual side-effects of many antidepressants. The number is the Odds Ratio (OR) compared to placebo, such that placebo is defined as 1. The numbers are rounded to a whole number. Data from the Supplementary Table from the paper above. http://links.lww.com/a1028
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||